Are your child’s immunizations up to date?
If you are the mother of an infant or toddler under three years old, your child may qualify to help study new or improved immunizations and their effectiveness.
Compensation is available.

Immunize Your Child From Preventable Diseases
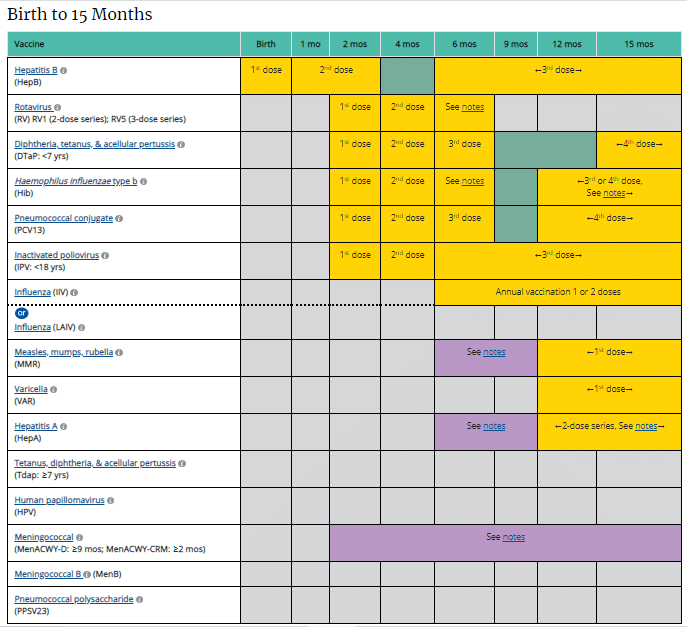
Immunization is the only effective method of preventing certain infectious diseases.
It protects most of the world’s children from diseases that previously caused millions of deaths each year. It can take days and weeks for a vaccine to help your child make protective antibodies to combat illness-causing preventable diseases. Some vaccines require multiple doses to provide the best protection.
Most parents choose to immunize their children. Sometimes families get busy and get behind in their immunization scheduling. The CDC (Centers for Disease Control and Prevention) recommends an immunization schedule to keep your children and others safe. Not receiving all doses of a vaccine may leave your child vulnerable to serious illness.
If your child misses any dose or gets behind schedule, you need to get your child immunized as soon as possible. Immunization is critical to protect your child. Most health insurances cover the cost of immunizations.
CTA: Participate in this immunization study.
Free Care and Compensation Available
You may qualify to help improve immunizations for your community and be compensated for your help.
Your child may be eligible for free recommended immunizations through our program.
What happens in this research study?

Help Others
Help others by contributing to medical research.

Early Treatment
Gain access to medical treatments before they are widely available..

Care and Compensation
Paid medical care during the study and compensation for your time and travel.
HOW IT WORKS
Eight Steps from One to Done
Initial conversation by phone or in the clinic with a study team member to tell you about the study opportunity
Review and complete a form that explains your rights and obligations as a participant in the study.
Do you meet the basic study requirements? Vitals, labs, medical history, exams, or other procedures may be conducted.
You are assigned to a treatment course, which could be a placebo. You and your doctor may not know which you receive – study treatment or control placebo.
These may be days, weeks, or months apart. The study protocol outlines what happens at each visit, such as labs, physical exams, questionnaires, or other tests/procedures.
You will discontinue the treatment of either the study treatment or the control placebo. You will also discuss your experience up to this point.
The study team may call you, or you may have occasional appointments to assess how you are doing following the study treatment.
Your participation in the study is complete. Often, you are asked to share your final feelings on the experience to drive improvements.
Ready to Make a Change?
Immunization protects children from severe sickness and complications of vaccine-preventable diseases. Immunize your child.
